Introduction
Your advisor has carefully monitored your progress in 5.301. You are ready to move from the technique modules to an actual project. This project will require you to use many of the skills that you have learned over the past three weeks to address a specific question. In addition, you and your lab mates will learn to work as a research group in order to reach a definitive goal in a short period of time.
Overview
Our research group has a longstanding interest in the synthesis of derivatives of penicillin, which is classified into the β-lactam class of antibiotics having a common structural backbone as illustrated in Figure 1.
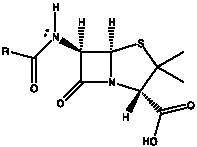
By changing the R group, we will synthesize a new penicillin derivative library, (a number of different R groups will be available)
Penicillin is used to treat many types of bacterial infections mostly Gram (+) and sometimes Gram (-) organisms. Since the discovery of penicillin there has been an ever-increasing resistance by the bacteria to many of the penicillin molecules presently on the market. In this research project, you will be synthesizing a new group of penicillin derivatives in order to overcome the antibiotic resistance to various strains of bacteria. Our research group will begin a comprehensive study, which will afford each student an opportunity to synthesize a fresh penicillin derivative. Go to the library and look through the literature, talk to the department graduate students on this project about the reactions that you will be running, and be sure to organize your efforts with your fellow lab mates.
In this research project you will each synthesize a penicillin derivative through acylation of 6-aminopenicillanic acid. This process is illustrated in Scheme 1 ( PDF).
PDF).
A wide selection of acyl chlorides will be made available to choose from. Progress of the reaction will be followed using TLC plates with additional characterization by NMR and MASS SPECTROSCOPY. The penicillin derivatives once synthesized will be purified by column chromatography and tested in a bioassay to determine and quantify their ability to kill bacteria. A broth dilution method will be developed to determine the concentration of penicillin that inhibits the growth of 50% of bacteria in vitro. Each of the penicillins will be tested using broth diluted with E. coli, a Gram (-) bacteria. After incubation of the diluted samples, optical density (OD) measurements will be made measuring their absorption at 600 nm on a Perkin-Elmer UV-VIS spectrometer. The results of the entire class will be made available to all participants so that the students in their final reports can develop structural and functional relationships among the various penicillin derivatives and discuss trends used in ranking the various derivatives based on their effectiveness at inhibiting bacterial growth. We will be synthesizing penicillins that may not yet be commercially available. The goal is to create and identify new antibiotics to stave off biological resistance by the bacteria.
What is unique about our project is the type of bacteria that we will be using. We have worked out the method and adapted it to (BL1) E. coli Gram (-) bacteria (Strain ATCC 10798). Since penicillin antibiotics are most effective against Gram (+) bacteria, we wanted to look carefully at the potential of their action against a Gram (-) strain. Our thinking here was that this is a less virulent strain to work with in terms of student safety (BL1) and secondly, if the results are promising for the Gram (-) strain we potentially could apply our most effective synthetic derivatives against the more dangerous Gram (+) bacteria in a (BL2) rated lab. See the MIT Undergraduate Lab safety considerations (PDF) for more information on our BL1 rated lab.
1 Adapted from: Whitaker, D. R., M. L. Truhlar, et al. Journal of Chemical Education 87 (2010): 634–6.
